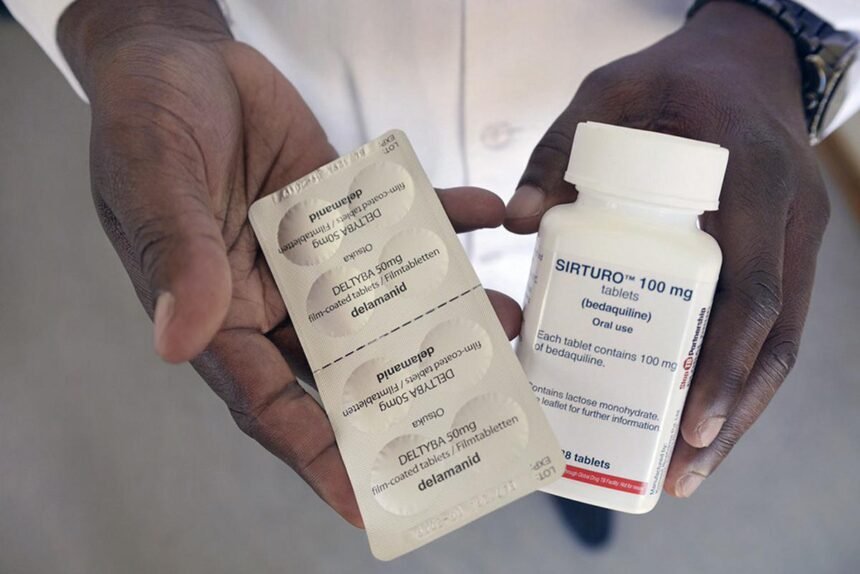
Johnson & Johnson, a multinational pharmaceutical company, had filed for an extension of its patent on tuberculosis (TB) drug bedaquiline in India.
Bedaquiline is an important drug used in the treatment of drug-resistant TB, which is a growing problem in India and other developing countries. The rejection of J&J’s patent extension means that other generic drug manufacturers will be able to produce and sell the drug at a lower cost, making it more accessible to patients in need.
The decision by India’s patent office is seen as a victory for public health advocates who have been pushing for greater access to affordable medicines for people in developing countries. However, J&J has expressed disappointment with the decision, stating that it could hurt future innovation in the field of TB drug development.
That’s great news for patients in India who rely on Bedaquiline for the treatment of multi-drug-resistant TB. The rejection of Johnson & Johnson’s attempt to extend its monopoly on the drug in India will pave the way for more affordable and wider access to this life-saving medication. This decision is particularly important because India has the highest burden of TB in the world and access to affordable drugs is critical in fighting the disease.
J&J’s practice of evergreening, which involves extending the life of patents to maintain revenue, has been ongoing since 2007. In the case of the patent on the fumarate salt formulation of Bedaquiline, TB survivors Nandita Venkatesan and Phumeza Tisile challenged the practice by filing a patent challenge in 2019. Their goal was to ensure that the safer, oral, and effective drug Bedaquiline would be available to all people who need it.